
One of the aims of the NHS Long Term Plan is that by 2028, three in four cancers (75%) will be diagnosed at an early stage. To achieve this, Humber and North Yorkshire Cancer Alliance works with partners on cutting-edge approaches to cancer diagnostics. This is made possible through innovative technology.
Innovation is a cornerstone of the NHS, and helps with many aspects of Cancer Alliance work, including:
- Operational pressures
- Increased demand
- Health inequalities
- Improving outcomes
With the help of our work, ground-breaking achievements in cancer diagnosis and treatments have been made possible. Find out more below.
Cancer Innovation Grants 2024/25
If you have an innovative project that promotes early cancer diagnosis, treatment, recovery and/or patient experience in the Humber and North Yorkshire area, Humber and North Yorkshire Cancer Alliance could help you with the funding.
The Cancer Innovation Grants form part of the Cancer Alliance’s aim to build a culture of cutting-edge cancer innovations, in line with the NHS Long Term Plan.
From small, grass-roots projects, to larger-scale innovation schemes, we’re encouraging the development or adoption of innovative approaches to cancer that target local priorities for improvement.
Click here for more information on the Cancer Innovation Grants 2024/25 programme.

Colon Capsule Endoscopy
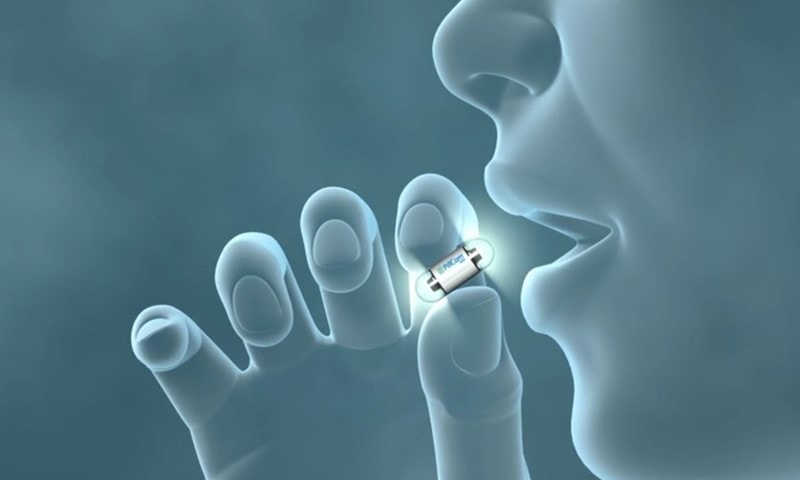
Colon Capsule Endoscopy (CCE) uses imaging technology within a small capsule – the size of a vitamin pill.
CCE is a less invasive alternative to a colonoscopy. Patients can swallow these cameras as they go about their day, while the camera pill takes pictures of the bowel as it passes through. The pictures are beamed to a recording device around the patient’s waist, and a diagnosis can be provided within hours.
York and Scarborough Teaching Hospitals Foundation Trust was one of 50 NHS sites to trial the Colon Capsule Endoscopy during a three-year pilot scheme. Patients who were referred to hospitals in the area that took part were able to receive faster diagnosis than through traditional methods.
Find out more
The CCE pilot was introduced at pace during the Covid pandemic. This was done to provide additional capacity for the investigation of symptomatic patients at risk of colorectal cancer.
These futuristic miniature cameras are a time- and labour-saving alternative to a colonoscopy. They can help rule out colon cancer and be used as a filter or triage test. This means therapeutic colonoscopy can be saved for people who need a biopsy or polypectomy.
If used in the right cohort of patients, CCE can help release endoscopy capacity and aid early cancer detection, by prioritising people in need of further tests and treatment.
Cytosponge
The Cytosponge is an innovative test, developed to identify Barrett’s oesophagus (BO). This is a condition that can increase the risk of developing oesophageal (food pipe) cancer.
A Cytosponge is a cell collection sponge contained within a swallowable capsule. After swallowing, the sponge is removed with the use of an attached thread by a trained nurse/clinician.
Learn more about the national Cytosponge trial.
Additional information
The Cytosponge is a capsule with a small sponge inside. The patient swallows the capsule, which has a thread or string attached.
The sponge is then released from the capsule, and a trained nurse/clinician pulls the thread back up, collecting small samples of cells. This is then sent to pathology for analysis.
The aim of the pilot study was to determine if offering the test to patients on medication for gastro-oesophageal reflux would increase detection of Barrett’s oesophagus. This condition is currently diagnosed via endoscopy following a GP referral to a gastroenterologist, or other specialist in secondary care.
Gastro-oesophageal reflux is a relatively common condition. Approximately 20% of the population experience symptoms, and some may already have or may develop BO, a precursor to oesophagus cancer (El-Serag et al., 2014).
A variety of demographic and lifestyle factors may increase the risk of gastro-oesophageal reflux, including:
- Old age
- Male sex
- Intake of analgesics
- Consumption of certain types of food and drink
- Smoking
- High body mass index (BMI)
- Limited physical activity
Endoscopy remains one of the most pressurised services in the NHS. Using Cytosponge as a triage tool in secondary care has the potential to release capacity of endoscopy services by discharging patients at low risk (routine and surveillance), and ensures patients at high risk can be prioritised effectively.
Non Specific Symptoms Pathway
Non Specific Symptom (NSS) Pathways – formerly known as Rapid Diagnostic Centres – are designed as urgent suspected cancer pathways. NSS cater to patients who don’t qualify for specific cancer pathways, but exhibit a range of vague symptoms (such as weight loss or fatigue), placing them at risk of a cancer diagnosis.
Since 2019, Cancer Alliances have developed new dedicated urgent diagnostic pathways for patients. The goal is to ensure every cancer patient with concerning but non-specific symptoms has the right tests at the right time, in as few visits as possible.
The development of new NSS pathways is a key priority, as is optimising the utilisation of existing ones. All Cancer Alliances are working to achieve 100% population coverage for patients exhibiting non-specific symptoms of cancer.
More information
Patients who present with non-specific symptoms or combinations of symptoms that potentially indicate various cancers often face problems. These challenges, associated with poorer patient outcomes, include:
- Delays in diagnosis
- Higher rates of late presentation
- Emergency presentation
Patients without established effective referral pathways may have:
- Seen their GP several times before referral
- Been more likely to attend in an emergency setting, such as a hospital accident and emergency department
- Presented with cancer at an advanced stage
- Been referred on to multiple pathways
- Gone back and forth between primary and secondary care
NSS pathways can be based in primary care, run by GPs, or in hospitals as a consultant or nurse-led service. Details of symptoms will be taken, as well as a range of tests, including:
- Blood test
- Chest X-ray
- CT scan
- Endoscopy
Such tests will rapidly progress patients to the most appropriate pathway. Less than 7% of patients referred to NSS pathways receive a cancer diagnosis. Approximately 45% have a non-cancer condition diagnosed, and don’t need to be referred back to their GP.
NSS services are in place across all Humber and North Yorkshire Trusts, and the roll-out of the NSS pathway is a core commitment of the NHS Long Term Plan. We’re working to ensure all patients with NSS will be referred via this pathway.
Lynch Syndrome
This syndrome is an inherited genetic condition that increases the risk of certain cancers, including:
- Bowel
- Ovarian
- Pancreatic
It’s estimated that one in 400 people in England (around 175,000 people) have Lynch syndrome. Only 5% are aware they’re living with the condition.
Our Lynch syndrome testing programme ensures patients at a heightened risk of cancer are offered surveillance and preventative treatments.

More details
Each year, 1,100 colorectal cancers are caused by Lynch syndrome. It’s the most common form of hereditary colorectal cancer, and around half of all people with Lynch syndrome develop this cancer.
The National Institute for Health and Care Excellence (NICE DG27 diagnostic pathway) recommends all people with colorectal cancer are tested for Lynch syndrome.
The national programme ensures everyone diagnosed with either bowel or endometrial cancer is offered genomic testing. A Lynch syndrome diagnosis helps with personalised cancer treatment and also means the patient’s relatives can be offered testing.
PinPoint

This test was developed by PinPoint Data Science, in collaboration with the University of Leeds and Leeds Teaching Hospitals NHS Trust, with the support of the Yorkshire and Humber Academic Health Sciences Network.
PinPoint is a new type of artificial intelligence (AI)-driven blood test, designed to help GPs to determine the likelihood of a patient having cancer. The test promises to deliver shorter referral waiting times, reduced patient anxiety and improved early cancer detection.
Find out more
PinPoint uses AI to examine features in blood and is currently being piloted by the NHS.
This diagnostic test was developed using the data of hundreds of thousands of patients investigated for cancer between 2011 and 2019. Through machine learning, 33 biomarkers of blood sample are measured. This information is combined with other data, such as age and gender, to generate a single number. This number reveals the chance of a patient having cancer.
PinPoint, when used in the earliest stages of clinical investigation, helps doctors determine within 72 hours the chance of a patient having cancer. They can then either prioritise the patient for hospital testing, or rule them out of the cancer pathway entirely.
Read frequently asked questions about the PinPoint test.
